Mass photometry measures antibody aggregation
A key quality attribute during antibody production is stability. Unstable antibody samples can aggregate, drastically reducing the bioactivity of therapeutics and compromising their safety by triggering immunogenic responses. Analytical tools that can efficiently and effectively characterize antibody stability are essential for successful antibody production.
Since mass photometry measures the mass of individual particles in solution in a matter of minutes and using only nanograms of sample, it can readily detect and quantify different aggregation species. Moreover, mass photometry is currently the only analytical technique that can directly measure antibody samples in the nanomolar concentration range, such as those found in IV bags.
The Antibody Stability Module automates antibody aggregation analysis
To complement the low turnaround times of mass photometry, Refeyn provides an Antibody Stability Module for its StreamlineMP software suite. Designed to handle large datasets obtained in antibody aggregation analyses, the module allows the user to easily compare different samples (e.g. antibody types, stress conditions). Thanks to the Antibody Stability Module, an analysis of a large dataset, which would take hours with manual analysis, can be completed in less than 20 minutes.
To learn how mass photometry streamlines antibody stability analysis
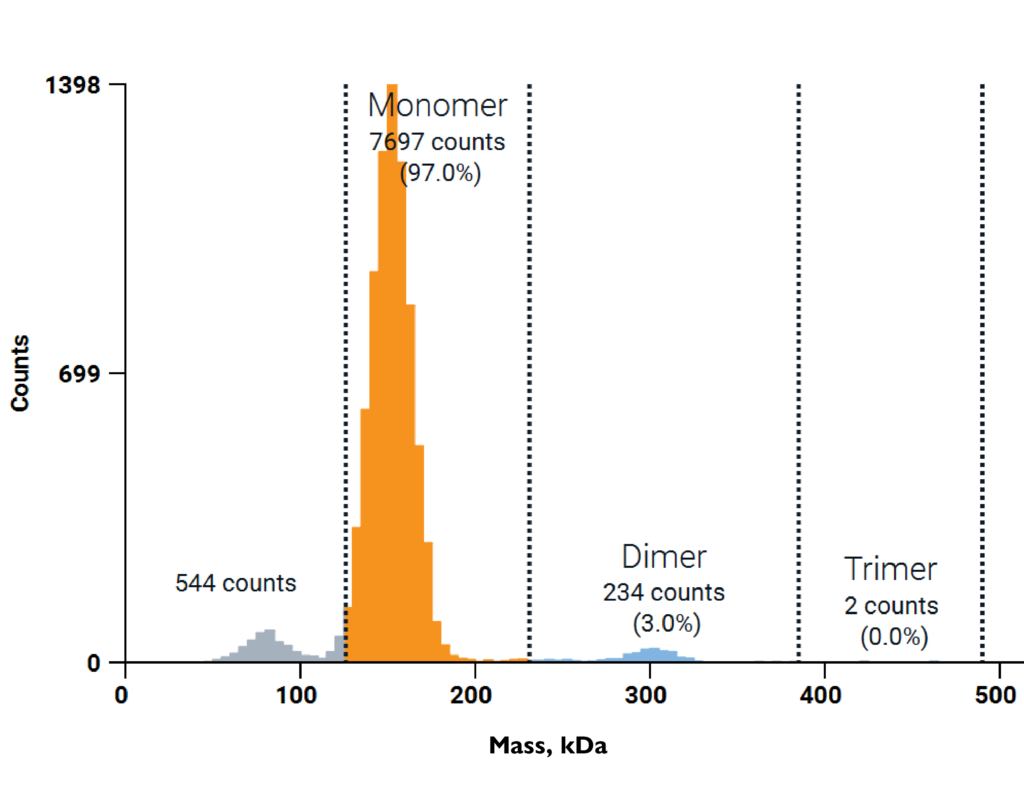
Quantifying antibody aggregation with the mass photometry Antibody Stability Module

In this technical note, you will see how to use mass photometry and the Antibody Stability Module to quickly detect and quantify antibody aggregation. The tech note also shows how mass photometry can measure antibody aggregation at both nanomolar and micromolar concentrations.
To demonstrate the application of mass photometry measurements, this tech note shows measurements of commercial antibody samples, as well as the results of a forced degradation study.
Additional resources on using mass photometry to characterize protein samples
TECH NOTE: Mass photometry vs. SEC in forced degradation studies
This tech note shows how mass photometry and SEC-UV compare when used to characterize the results of forced antibody degradation studies. We demonstrate sample analysis through two case studies: By RIC biologics (Kortrijk, Belgium) and the University of Oxford’s Kavli Institute for Nanoscience Discovery.

WEBINAR: Rapid analytical approach for biologics characterization
In this webinar you will learn – via example data and case studies – how mass photometry supports the development and production of therapeutic biologics such as antibodies and AAVs. Mass photometry is a powerful bioanalytical tool that characterizes samples at the single-molecule level with low sample consumption, fast turnaround times and no need for labels. These strengths make mass photometry an ideal technique to characterize key analytical attributes of therapeutic biologics at different stages of development and production.
To find out how mass photometry can accelerate antibody production
More Application Notes
Browse through our catalogue of application notes highlighting some recent case studies featuring mass photometry.
