Characterize larger viral or VLP vectors, providing information on size, purity, and stability within minutes.

The KaritroMP makes it easy to quickly characterize samples of large viral vectors and virus-like particles (VLPs). Leveraging innovative macro mass photometry technology, the KaritroMP’s unique functionality gives it an edge in terms of speed, ease of use and the information it provides.
Through single-particle analysis, the KaritroMP enables users to visualize the distributions of particles present in their samples in minutes. Measuring two distinct parameters for every particle, it resolves distinct populations more effectively than other techniques.
Just as Refeyn’s SamuxMP mass photometer returns game-changing AAV analytics in just minutes and the TwoMP mass photometer offers fast and effective RNA analytics, the KaritroMP quickly returns valuable new insights into the purity, stability and composition of large viral vector samples. The Karitro joins the Samux and TwoMP to create a powerful analytical toolbox for cell and gene therapy, and vaccine development – for every modality.
Characterization of large viral vectors and VLPs
Semi-automated workflow
Fast and easy to use

Purity
Stability
Size distribution analysis
6 minutes per sample
Minimal sample prep
Cost-efficient operation with ready-to-use consumables
Little sample needed (5 μL at 1E8 – 1E9 particles/mL)
Benchtop instrument
Insights at a glance
For each particle in a sample, the KaritroMP measures two distinct parameters: Contrast (a qualitative measure related to the particle’s composition and mass) and size. For further details about how macro mass photometry works, see our overview of macro mass photometry technology.
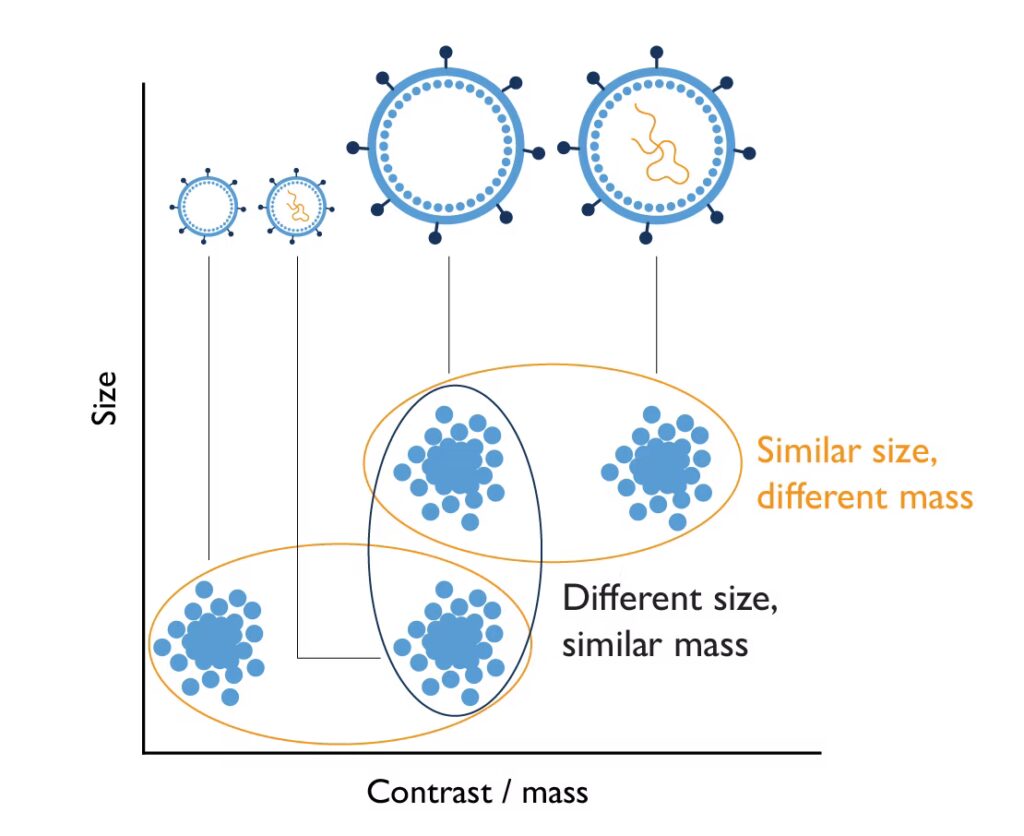
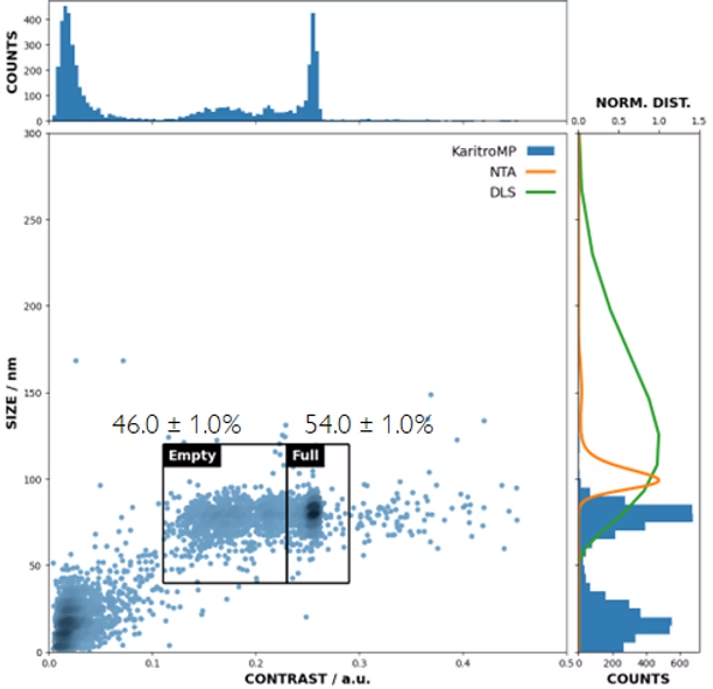
Because it measures contrast and mass, the instrument can distinguish populations of particles with the same size but different mass. For example, it can resolve empty vs. full adenovirus capsids and quantify their proportions (Fig. 1).
Comparing data from the KaritroMP with dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) shows that only the Karitro could resolve the empty and full populations. With both NTA and DLS, only one population was apparent.
Its speed and excellent contrast resolution make the KaritroMP the only instrument on the market that can quickly and directly measure AdV empty-full ratios.
Enjoy greater reproducibility across your measurements with SizeFerence, a calibrant for the KaritroMP. SizeFerence provides reliable calibrations for macro mass photometry measurements of particles in the 40–150 nm range, including adenoviruses, lentiviruses and VLPs. Contact us to learn more and order now.