Mass photometry supports membrane protein purification protocols
Membrane protein purification and membrane mimetics
Integral membrane proteins are promising targets for both basic and translational research. These proteins are embedded in a lipid bilayer in their native environments, from which they need to be extracted prior to the study. As the transmembrane domains of membrane proteins are hydrophobic, it is important to be using membrane mimetics which helps with solubilization in aqueous buffers. Therefore, membrane mimetics such as detergents, nanodiscs or SMALPs, enable characterization studies of membrane proteins.

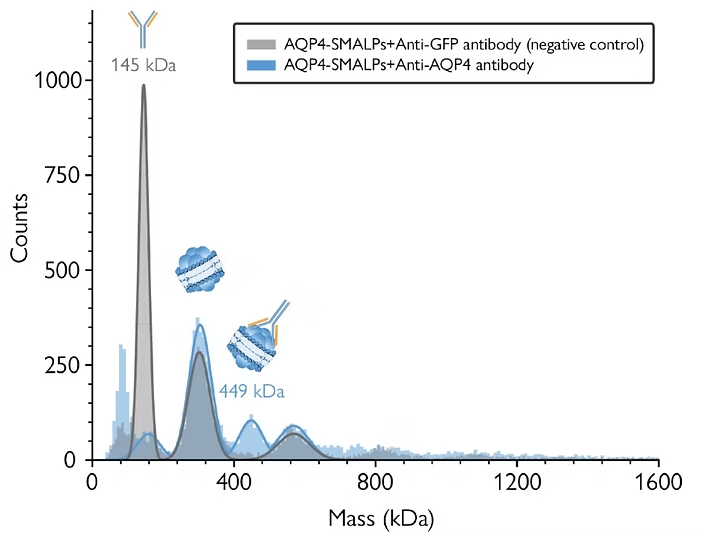
Rapid characterization of membrane protein samples
Mass photometry is a powerful bioanalytical technique that provides detailed information on the mass distribution of a sample. Importantly, mass photometry measurements are fast, require little sample, and are largely compatible with membrane mimetics like nanodiscs, detergents or SMALPs, making the technique an excellent addition to membrane protein purification processes.
Mass photometry characterizes samples at the single-molecule level, which allows it to describe complex samples with multiple components, including rare species. This makes it particularly useful during the purification process, as it can readily detect aggregation and impurities.
To read how mass photometry can help you characterize membrane protein samples
Characterization of nanodisc-embedded membrane protein samples with mass photometry
In this application note you will see how mass photometry can be used to support membrane protein purification, and how it can measure and characterize samples of membrane proteins embedded in nanodiscs. You will read a real case study of mass photometry applied to the purification process of a membrane protein Aquaporin-4, solubilized with SMALPs. Finally, you will see the results and interpretations of mass photometry analyses performed at different stages during the purification process – including size-exclusion chromatography (SEC) purification and antibody-based assays.
Additional resources
WEBINAR: Investigating membrane protein complexes with mass photometry
This webinar shows the applications of mass photometry for the study of two examples of macromolecular complexes formed by eukaryotic membrane proteins. In both cases, mass photometry was crucial to addressing highly relevant physiological questions. The webinar showcases what value can mass photometry deliver, and what makes it suitable for studies of membrane protein complexes.

BLOG: How to study membrane proteins with mass photometry
Mass photometry provides valuable information on the purity and behavior of samples containing membrane proteins and membrane mimetics. In this blog post, we examine the role of mass photometry in membrane protein analysis and describe case studies that illustrate how mass photometry is contributing to the field.
To learn more about how mass photometry streamlines membrane protein analysis
More Application Notes
Browse through our catalogue of application notes highlighting some recent case studies featuring mass photometry.
